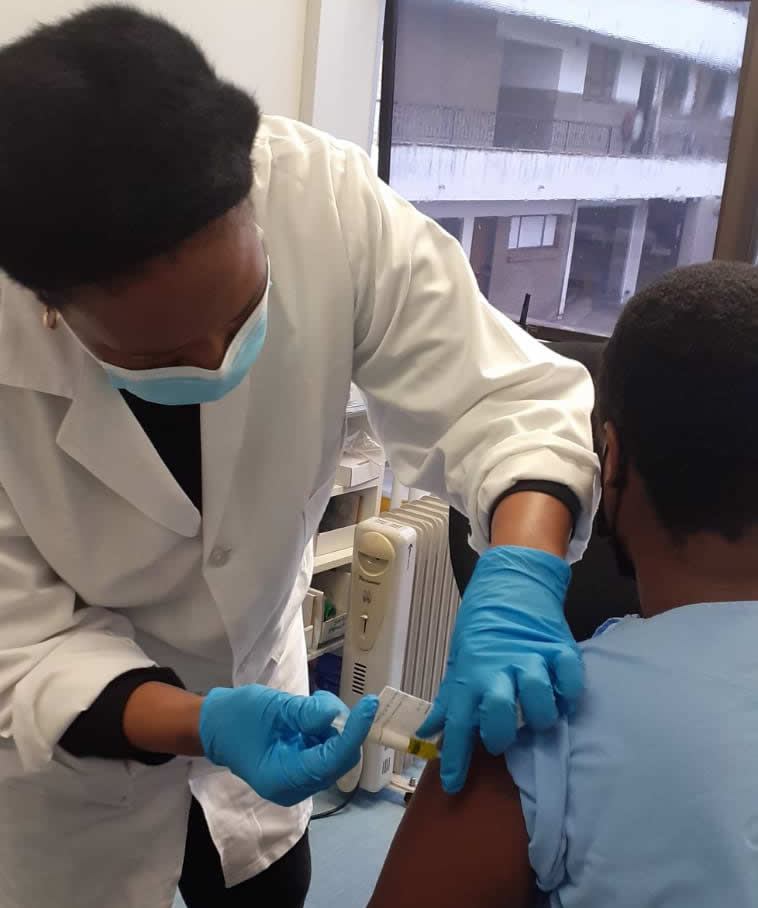
While South Africa and the rest of the world continue to make encouraging progress in their response to the COVID-19 pandemic, the development of a safe and effective HIV vaccine also remains critical for global health – something the South African Medical Research Council (SAMRC) has not lost sight of.
Through its HIV Prevention Research Unit (HPRU), the SAMRC recently enrolled the first participant in South Africa for the groundbreaking PrEPVacc Trial at its Verulam Clinical Research Site in Durban, KwaZulu Natal. This historic enrollment brings the number of sites enrolling into the PrEPVacc study, to four – including others in Masaka, Uganda and Mbeya and Dar es Salaam in Tanzania.
PrEPVacc, a double blinded randomized clinical trial, is an African-led, European-supported HIV prevention study running in East and Southern Africa from 2018 to 2023 – for the first time, it is combining the evaluation of experimental HIV vaccines and pre-exposure prophylaxis (PrEP) simultaneously. In this Phase IIb HIV prevention trial, all participants will be offered oral PrEP in the initial phase of the study together with HIV Vaccine. This provision of PrEP is to compare efficacy of oral Truvada against Descovy as pre-exposure prophylaxis – the outcome will tell us whether the regimens can protect against HIV infection.
Like most first-time clinical trial participants, a young woman who resides with her mother, grandfather, and seven-year-old daughter in the north of Durban, was excited as she signed the consent form to volunteer to be in the HIV prevention vaccine trial. However, she told Sihle Mkhamisa, Verulam’s site recruiter that she was anxious initially about blood draws in the trial but after talking to Sihle, the health counsellor and study nurse, she felt more reassured and less anxious, and voluntarily decided to enroll in the study. “I want to help prevent HIV infection in my community,” the shy young woman told Neetha Morar, senior research manager at HPRU – also adding that she is motivated to join the study and would talk to others in her community about her experience in the trial soon.
For the first enrollment at the Verulam Site, the study products (vaccines and pills) were administered by study Nurse Nana Mtambo and verified by Nurse Nalini Naidu.
Dr Nishanta Singh, study site Principal Investigator (PI) extended special thanks and gratitude to all the study participants, community members, the PrEPVacc research team, its sponsors, and partners for their support in preparing for the trial to begin in South Africa.
Echoing these sentiments, Dr Eugene Ruzagira, Trial Director of PrEPVacc who is based at the MRC/UVRI & LSHTM Uganda Research Unit, also congratulated all the staff and participants and supporters at Verulam on beginning enrolment to PrEPVacc. “It has taken a great deal of commitment and hard work to prepare for this HIV prevention study that is testing two ways to prevent HIV at the same time, and we are very pleased to have begun the clinical trial in South Africa,” said Dr Ruzagira.
PrEPVacc aims to enroll 1,668 participants across four sites in Africa collectively by June 2022 with follow up expected to continue until 2023. PrEPVacc is funded by the European & Developing Countries Clinical Trials Partnership (EDCTP), as part of the EDCTP2 Programme supported by the European Union.
Are you interested in being part of PrEPVacc? | Read more