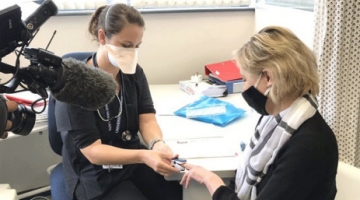
Professor Gray, who is the CEO and president of the Medical Research Council, was screened and vaccinated at UCT’s Lung Institute on Tuesday.
CAPE TOWN - One of South Africa’s most respected physicians, Professor Glenda Gray, is participating in a COVID-19 vaccine study to show anti-vaxxers that it’s safe.
Professor Gray, who is the CEO and president of the South African Medical Research Council, was screened and vaccinated at UCT’s Lung Institute on Tuesday.
Since June, South Africa has been collaborating with the UK’s Oxford University in the randomised control study that’s also running in Britain and Brazil.
South Africa is now well into the race to find a vaccine that will immunise against COVID-19.
The country has five sites screening and enrolling participants in the ChAdOx1 nCoV-19 randomised control trial.
Gray has been vaccinated at the UCT Lung Institute site, as one of more than 1,000 participants enrolled so far.
“Normally I am at the other end of the needle. For me it was very important to be a participant. To see what it’s like, have them rake your blood, get the injection. It’s critical that we show that COVID-19 affects everybody.”
The ChAdOx1 trial vaccine was made by using genetic material called spike glycoprotein, to mimic the properties of SARS-COV2 - the actual coronavirus that causes COVID-19.
Scientists are aiming to prepare the human body to recognise and develop an immune response to the spike glycoprotein and block the SARS-CoV-2 virus from entering human cells.
Original article posted on EWN.