South Africa’s first Covid-19 Antigen Self-test launched
Cape Town, South Africa, - In mid-2020, the South African Medical Research Council SAMRC) rallied key local partners from government, academia, and industry to help reduce the country's reliance on international test kit supplies through the local development and manufacture of robust alternatives capable of producing results before patients leave the testing site.
With the guidance of the National Health Laboratory Service and others, the SAMRC, together with Department of Science and Innovation (DSI) and the Technology Innovation Agency (TIA), a DSI entity, jointly ran a call for applications to identify suitable projects for funding. The development of the first Medical Diagnostech Covid-19 antigen test was co-funded through this mechanism.
Today, the Medical Diagnostech (Pty) Ltd announces the launch of South Africa’s first Covid-19 Antigen Self-test with companion mobile phone application, HealthPulse TestNow. The company developed the rapid diagnostic test, while the application was developed by the Seattle-based company, Audere. The application provides detailed instructions on how to perform the self-test and assists in interpreting the results through image capture of the rapid test device.

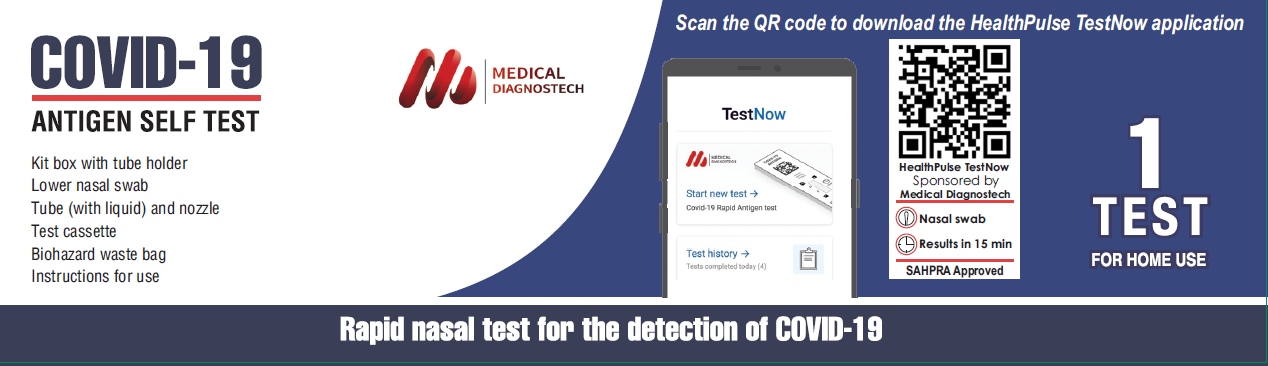
HealthPulse TestNow is the first Covid-19 app deployed in South Africa that supports self-testers with the administration and interpretation of rapid diagnostic tests (RDT). HealthPulse TestNow helps ensure the accurate use of RDTs through easy-to-follow instructions, process control timers, and guided result interpretation. The solution seamlessly integrates with public health reporting systems and ensures that self-testing data is reported, providing a more comprehensive understanding of disease prevalence.
Self-testing an important component of public health strategies worldwide, however, challenges exist with accurate and reliable test administration, interpretation, and collection of data that can compromise the benefits of these efforts. HealthPulse TestNow is designed to improve an individual’s self-testing aptitude while seamlessly connecting ministries of health with test data in an effort to maximize the end-to-end impact of public health programs.
“We are proud to announce the first deployment of HealthPulse TestNow in South Africa. While the current state of Covid-19 testing varies across communities, this AI-powered digital solution lays the foundation for a much-needed self-testing tool that facilitates improved self-diagnosis and early access to care not just for Covid-19, but for a range of medical conditions. We are actively expanding HealthPulse TestNow's coverage to include new conditions across a broader set of geographies in Africa. Our mission is to empower early diagnosis and expedite access to care.”
- Dr. Dino Rech, CEO, Audere
The current announcement comes on the heels of the approval of the company’s rapid diagnostic test on 28 March 2023 by the South African Health Products Authority (SAHPRA).
The rapid test and companion application were subject to rigorous evaluations by the National Reference Laboratory (NRL) of South Africa, the National Health Laboratory Service (NHLS). The NHLS also conducted multi-provincial clinical trials to determine the rapid test kit’s usability amongst lay persons.
“We are thrilled to introduce this new product to South Africans and the rest of Africa in due course,” said CEO of Medical Diagnostech, Ashley Uys. “South African healthcare professionals and patient groups have been asking for new and innovative medical products and we believe that the MD Covid-19 Antigen Self-test is the catalyst to more innovative biomedical and diagnostic products”.
This is just one of many products in Medical Diagnostech’s wide range of diagnostic products and the company plans to continue pushing the envelope within the health market with future releases.
MD Covid-19 Antigen Self-test will be available at all major pharmacies.
For more information about Medical Diagnostech, please visit: www.medi-tech.co.za.
To arrange an interview with Ashley Uys, CEO of Medical Diagnostech (Pty) Ltd, please contact the company at +27 21 982 0673 or email info@medi-tech.co.za.
About Medical Diagnostech (Pty) Ltd
Medical Diagnostech was established in 2010 as a developer and manufacturer of lateral flow rapid diagnostic test kits. The Cape Town-based company manufactures high quality rapid diagnostic test kits using its secret methodology for increased sensitivity and early detection. Medical Diagnostech’s products are robust and are optimized to withstand extreme storage conditions for up to 24 months. Lateral flow tests are manufactured under ISO 13485 accreditation and includes tests for alcohol consumption, drugs of abuse, HIV, malaria, pregnancy, fertility/ovulation and Covid-19.
About Audere
Audere is a global digital health non-profit developing HealthPulse™ solutions to advance health equity in underserved communities worldwide. The organisation operates at the unique intersection of global health and high tech, creating advanced, accessible software that revolutionizes the detection and treatment of diseases — such as malaria, COVID-19, and HIV. Audere’s diverse team of passionate, innovative minds combines smartphone technology, artificial intelligence, and the best of cloud-based services to deliver HealthPulse™ solutions worldwide. Development of the company’s projects is funded by grants and support from the Bill & Melinda Gates Foundation, FIND, and other global health partners. Learn more at www.auderenow.org.
Contacts
Medical Diagnostech (Pty) Ltd
Averouz Maritz
Quality Assurance & Regulatory Affairs
+27 21 982 0673
info@medi-tech.co.za
Audere
Jennifer Thorson
Marketing
+1 425 835 3801
info@healthpulsenow.org