Cochrane South Africa
News & Events
| Cochrane South Africa Hosts Three-Day Evidence-to-Decision Protocol Development Workshop |
|
Cochrane South Africa (CSA) recently hosted a three-day Evidence-to-Decision (E2D) Protocol Development Workshop, aimed at strengthening national capacity for evidence-informed decision-making within the South African health sector, taking place at the South African Medical Research Council’s (SAMRC) Conference Centre from 18-20 February 2026. The E2D project is a Department of Health-funded initiative in partnership with the SAMRC’s Health System Research Unit, Cochrane South Africa and Stellenbosch University’s Centre for Evidence-Based Health Care. The workshop formed part of the work of the E2D Goal 3 Training Working Group, which focuses on building the skills of both evidence producers and users who contribute to evidence-informed decision-making (EIDM) and improved healthcare outcomes in the public health system. The training was facilitated by CSA’s Unit Director Prof Mark Engel and Senior Scientist Ameer Hohlfeld, who guided participants through a structured, hands-on learning process over the three days. Participants included Dr Janine Jugathpal, Deputy Director: Essential Drugs Programme (EDP) and members of the EDP Oversight Group of the Affordable Medicines Directorate, National Department of Health. The EDP Oversight Group is responsible for the review and updating of the National Standard Treatment Guidelines and Essential Medicines List and oversees the work of the ministerially appointed National Essential Medicines List Committee (NEMLC) and Expert Review Committee of the NEMLC. Workshop goals The primary goal of the workshop was to strengthen participants’ capacity to develop high-quality, methodologically sound protocols for evidence synthesis, aligned with the needs of decision-makers in the South African National Health Sector. Specifically, the workshop aimed to:
Programme activities and highlights The three-day workshop combined short lectures with highly interactive and practical learning activities, enabling participants to apply concepts directly to their own topics. Day 1 focused on laying the foundations of evidence-based health care and protocol development. Participants explored what makes EBHC distinctive, worked in groups to turn health system problems into structured research questions, and refined these questions through guided exercises. Interactive activities included outcomes prioritisation using a “fishbowl” approach, structured work on protocol rationale and objectives, and group reflections at the end of the day. Day 2 shifted towards the technical components of evidence synthesis protocols. Sessions covered eligibility criteria, data management, and the development of reproducible search strategies. Participants engaged in hands-on searching exercises, learned about article management using Rayyan software, and were introduced to the Risk of Bias 2.0 tool through guided practice. The day concluded with dedicated time for group protocol development and reflection. Day 3 addressed the later stages of the evidence synthesis process. Practical exercises focused on data extraction, synthesis essentials, handling heterogeneity, and applying the GRADE approach to develop Summary of Findings tables. The workshop concluded with final reflections and feedback from participants. Reflections from partners and facilitators Dr Jugathpal said, “We welcome the opportunity to strengthen capacity development through Cochrane SA, our partner in the Evidence to Decision Collaboration, thereby investing in the knowledge and skills required to ensure that the best available research evidence informs our national health policy and healthcare guidance”. “We consider it a privilege to be working closely with our colleagues from the Department of Health, in contributing to capacity strengthening for evidence-informed decision-making,” added Prof Engel. Strengthening evidence-informed decision-making By combining methodological rigour with practical, applied learning, the E2D Protocol Development Workshop contributed to strengthening the link between evidence generation and decision-making in South Africa’s health system. The workshop also reinforced Cochrane South Africa’s ongoing commitment to supporting high-quality evidence synthesis that responds to national health priorities. See some moments from the E2D Workshop | HERE |
| Ameer Hohlfeld participates at JBI Global Evidence Conference |

Ameer Hohlfeld, a Senior Scientist at Cochrane South Africa attended the JBI Global Evidence Conference in Kochi, India from 19-21 November 2025. The JBI Global Evidence Conference is a major international gathering focused on evidence synthesis, evidence implementation, and Knowledge Translation (KT) and brings together guideline developers, policymakers, researchers, clinicians, and KT practitioners who collectively influence how high-quality evidence is produced and used throughout health systems. The event is central to global efforts aimed at reducing research waste, improving evidence standards, and ensuring that decisions are informed by the full body of available evidence. “I presented two papers on publication bias. My aim was to contribute to global discussions on reducing research waste in evidence synthesis and clinical trials, while engaging with experts working to strengthen transparency, methodology, and knowledge translation. The conference provided an important platform to share our work and learn from international leaders advancing more trustworthy and accessible evidence practices”, said Hohlfeld. This year’s conference highlighted several critical topics and the themes which included: reducing bias, improving transparency across the research pipeline, and strengthening the systematic use of evidence in decision-making. Sessions on guideline development emphasised rigorous evidence synthesis, meaningful stakeholder engagement, and prioritising relevant research questions to avoid unnecessary duplication. The programme also showcased practical tools and methods, including the GRADE approach and strategies for adapting and contextualising guidelines for diverse settings. Innovations in KT—such as infographics and storyboarding—were presented as effective ways to make evidence more accessible to clinicians, policymakers, and communities. A major strength of the conference was the diversity of attendees. Participants included global leaders from JBI, evidence-synthesis specialists, clinicians, policymakers, journal editors, implementation scientists, and researchers at various career stages. Delegates came from Australia, Austria, South Africa, Italy, the Czech Republic, India, Bangladesh, the United Kingdom, Canada, the United States, Japan, Kenya, Malaysia, Nigeria, and many other countries across Europe, Asia, and Africa. This mix ensured rich discussions on how different health systems adapt evidence methods to their local contexts, especially in low- and middle-income countries. Outcomes of the meeting will inform several future directions, such as strengthening capacity-building activities, expanding training opportunities, and integrating new KT tools—such as infographics—into our departmental strategy. Lessons learned will also feed directly into efforts to improve transparency and prevent research waste. There is also interest in developing mentorship pathways, building toolkits for KT and implementation, and exploring sustainable funding models to allow wider access to evidence-based practice training. “Presenting in Kochi was energising and meaningful. Engaging with global experts deepened my understanding of research waste, publication bias, and KT, while affirming the importance of collaborative, transparent methods. The conference strengthened my commitment to advancing ethical, high-quality research and expanded my network of colleagues working to improve the impact of evidence across health systems”, concluded Hohlfeld. |
| Dr Jaca participates in WHO SAGE Typhoid Working Group meeting |
|
Dr. Anelisa Jaca, a Specialist Scientist at Cochrane South Africa participated in the World Health Organisation (WHO) SAGE Typhoid Working Group meeting which was held at WHO Headquarters in Geneva, Switzerland from 17–20 November 2025. The meeting focused on evaluating and updating policy guidance on typhoid conjugate booster vaccines (TCVs). During the sessions, the group reviewed the latest scientific evidence on TCV effectiveness and formulated recommendations for booster use. The Secretariat also presented key knowledge gaps related to typhoid epidemiology, vaccine efficacy, and optimal immunisation schedules. In addition, the working group identified priority research areas to strengthen future collaboration between the WHO Headquarters and Cochrane South Africa. Dr Jaca said, “It was a great honour to be part of this high-level decision-making process, and I look forward to continued collaboration in addressing the research gaps highlighted during the meeting.” |
| Cochrane South Africa Hosts Africa CDC Delegation to Strengthen Evidence Synthesis and Policy Translation Across Africa |
|
The South African Medical Research Council’s (SAMRC) Cochrane South Africa (CSA) hosted a high-level delegation from the Africa Centres for Disease Control and Prevention (Africa CDC), Science and Innovation Directorate, for a strategic engagement aimed at strengthening evidence synthesis, knowledge translation, and policy-informed decision-making across the continent. The engagement forms part of ongoing collaborative efforts led by Dr Duduzile Ndwandwe, a Specialist Scientist, whose work with Africa CDC continues to position CSA as a trusted technical partner in advancing public health evidence systems in Africa. The meeting was officially opened and spearheaded by Dr Ndwandwe, alongside the SAMRC Chief Research Operations Officer (CROO), Dr Mongezi Mdluli, signaling strong institutional commitment to regional collaboration and innovation in evidence-informed health policy. The Africa CDC delegation was led by Dr Nebiyu Dereje, who delivered an overview of the Africa CDC Science and Innovation Directorate, followed by a presentation on the Knowledge Management and Policy Translation Division. These presentations highlighted Africa CDC’s strategic priorities in research coordination, data-driven policymaking, and strengthening response systems across Member States. An overview of Cochrane South Africa’s Vision and Mandate was presented by CSA Unit Director, Prof Mark Engel, highlighting the unit’s leadership in evidence synthesis (qualitative and quantitative), capacity-strengthening initiatives, rapid evidence synthesis in response to outbreaks in Africa, and policy support. Contributions from Mr Ameer Hohlfeld, Dr Sara Cooper, and Dr Anelisa Jaca provided insights into the Evidence-Informed Decision-Making (EIDM) landscape in Africa, showcasing CSA’s role in strengthening policy-relevant research and rapid evidence responses. View the complete Cochrane South Africa Hosts Africa CDC Delegation to Strengthen Evidence Synthesis and Policy Translation Across Africa - Article |
| CSA staff exhibit at 17th International Federation on Ageing Conference |
|
For more than three decades the IFA Global Conferences on Ageing have attracted delegates from government, NGOs, industry, academia and across all fields of public health for powerful conversations that fuel action to improve the quality of life as part of protecting and advancing of the rights and dignities of older people. CSA’s Unit Director Prof Mark Engel, and Ameer Hohlfeld (Methodologies Research Portfolio lead), presented a 90-minute symposium at the conference, with the session entitled “Systematic Review Primer”. Attended by delegates which included colleagues from Jamaica and Canada, Engel and Hohlfeld explained the elements of reading and conducting systematic reviews with a worked example from the Cochrane Library on interventions to prevent falls in the elderly. Prof Engel said, “We were encouraged by the positive feedback from the attendees, who mentioned it was a worthwhile session, demystifying the systematic review process”. |
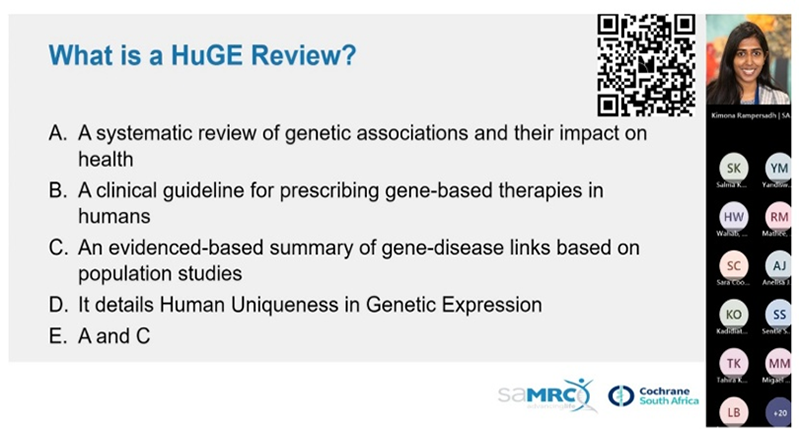
| CSA hosts webinar on Synthesising Evidence from Human Gene-Disease Association Studies |
|
Dr Rampersadh is a research fellow at CSA, where she focuses on synthesising evidence on genetically associated diseases to inform public health policy and practice. She holds a PhD in Medicine from the University of Cape Town and has a background in medical microbiology, with her research spanning antimicrobial resistance, Group A Streptococcus and rheumatic heart disease, employing both laboratory and epidemiological approaches. During the webinar, participants were introduced to methods employed in Human Genome Epidemiology Network (HuGE) reviews as a key apporach for evaluating gene-disease association studies, with an emphasis on their role in strengthening evidence reliability and guiding future research. HuGENet was established by the CDC Office of Public Health Genomics to aid in the translation of genetic research findings into opportunities for preventive medicine and public health by advancing the synthesis, interpretation, and dissemination of population-based data on human genetic variation in health and disease. The learning objectives included: understanding the purpose and value of HuGe reviews, an introduction to the HuGENet™ HuGE Review Handbook, and learning how to conduct HuGe reviews, illustrated through exemplars of published studies. View the HuGe Navigator | HERE Access the HuGeNet HuGe Review handbook | HERE Watch the webinar | HERE |
| CSA Hosts 2-Day Workshop to Strengthen Evidence-Based Health Research in South Africa |
|
Cochrane South Africa’s (CSA) Research Methods Portfolio team, under the leadership of Ameer Hohlfeld hosted a successful two-day workshop on the 17-18 September 2025 the South African Medical Research Council’s (SAMRC) Conference Centre, bringing together post-graduate students and researchers from within the SAMRC and universities across the country, including University of Limpopo, Stellenbosch University, University of the Western Cape, University of Cape Town, University of Johannesburg, and University of Zululand. The training highlighted the importance of evidence-based methods in preparing synthesis which will impact decision-making in improving health outcomes for all South Africans. CSA’s work focuses on acquiring high-quality, evidence-based information to inform health policy and practice, ultimately improving the quality of life and health status of communities across the nation. The workshop underscored CSA’s ongoing commitment to capacity building by equipping the next generation of researchers with the tools to conduct systematic reviews and contribute to evidence-informed healthcare. During the sessions, participants were introduced to the Cochrane Collaboration and library, while also receiving practical training from CSA staff, covering key concepts such as the importance of evidence-based healthcare, defining precise research questions, designing effective search strategies, conducting critical appraisals, evaluating studies, and registering protocols on international databases such as PROSPERO. The workshop also included a session on qualitative evidence synthesis (QES), led by Dr Sara Cooper, portfolio lead of the QES Hub at SAMRC, which provided participants with an introduction to QES principles and methods together with hands-on support for formulating an appropriate QES question. The event provided a vital platform for knowledge sharing and skills development, with several participants noting the direct value of the workshop in contributing to their own research projects. “The positive feedback received reflects the value of the content, as well as the appropriateness and necessity of CSA’s workshops in advancing evidence-based knowledge generation, while supporting and training post-graduate researchers to contribute meaningfully to South Africa’s healthcare landscape”, said Prof Mark Engel, CSA Unit Director.
|
| Cochrane South Africa present at 19th Vaccine Congress in Japan |
|
The Vaccine Implementation Research Portfolio at the South African Medical Research Council’s (SAMRC) Cochrane South Africa (CSA) leads innovative research which bridges the gap between vaccine development and real-world public health outcomes, with focuses on improving vaccine delivery systems, addressing structural barriers, and enhancing equitable access across Africa. CSA staff, Ms. Lindi Mathebula (Project Manager) and Dr Anelisa Jaca (Specialist Scientist) attended the 19th Vaccine Congress, held at the International Conference Centre in Kyoto, Japan, from 7-10 September 2025. The conference was organised by Elsevier Vaccine, one of the top journals in vaccine science, bringing together leading experts and researchers from around the world. The congress provided a crucial platform for sharing innovative discoveries and fostering new collaborations within the field, with the event showcasing the latest research in vaccine science through invited plenary lectures, contributed talks, and poster sessions.
“The marked increase in publications after 2022 indicates a growing interest in this field, but much of the research remains fragmented and narrow in scope. Addressing these gaps is vital for developing equitable and context-sensitive HPV vaccination strategies that not only target adolescents but also engage the broader network of influencers and decision-makers. A more inclusive evidence base will be critical to achieving meaningful progress in vaccine uptake and ultimately reducing the burden of HPV-related diseases in low- and middle-income countries. The research highlights significant gaps in HPV vaccine acceptance studies in Africa, emphasizing the need for broader representation beyond adolescent girls and for a more geographically diverse evidence base”, added Dr Jaca. These concerns resonate with discussions at the 19th Vaccine Congress, which stressed the importance of inclusive and context-sensitive vaccination strategies. A noteworthy initiative is the collaboration between the Organization of African First Ladies for Development (OAFLAD) and the Sabin Vaccine Institute to develop a cervical cancer elimination strategy. This partnership aims to strengthen HPV prevention efforts across the continent, demonstrating a commitment to engaging diverse stakeholders in vaccination advocacy. With its comprehensive agenda, covering everything from vaccine development to real-world uptake, the congress offered a platform conducive to fostering a spirit of collegiality and constructive feedback. It served as an excellent forum for engaging with the vaccine community, participating in discussions, and sharing ideas. More about the 19th Vaccine Congress | HERE More about CSA’s Vaccine Implementation Research | HERE |
| SANCTR to Shine at SACRA Conference 2025: Two Days of Innovation, Impact, and Insight! |
|
The South African National Clinical Trial Registry (SANCTR) team is set to make a splash, with a dynamic presentation on the theme “Technology & Data Sharing” presented by Dr Duduzile Ndwandwe, followed by a panel discussion featuring Ms. Thobile Malinga, and their very own exhibition stand. SANCTR is hosted within the South African Medical Research Council’s (SAMRC) Cochrane South Africa (CSA). What’s on the Agenda?
Why It Matters SACRA's “Partnering for Impact” theme perfectly aligns with SANCTR’s mission to elevate clinical research capacity, connect stakeholders, and pave smoother paths from protocol to patient. With enhanced networking, larger exhibition zones, and an emphasis on collaboration, this year’s conference offers the ideal stage for SANCTR to amplify its voice and showcase its initiatives. What You Can Expect
More information on the SACRA Conference 2025 | HERE Read more about SANCTR | HERE |
| Dr. Duduzile Ndwandwe attends WHO AFRO Regional Immunisation Meetings |
|
Dr. Duduzile Ndwandwe, Head of Vaccine Implementation Science at the South African Medical Research Council’s (SAMRC) Cochrane South Africa (CSA), recently participated in the WHO Regional Office for Africa (AFRO) immunisation meetings held from 24–27 June 2025 in Kinshasa, Democratic Republic of the Congo. She emphasised the urgent need for stronger partnerships and evidence-based implementation to further immunisation goals across Africa. Dr. Ndwandwe participated in the Regional Immunisation Stakeholders Meeting, where she was part of a high-level panel addressing the theme: “Strengthening partnerships to accelerate the implementation of the Immunisation Agenda 2030: What can be done differently?” In her presentation, titled “Advancing Partnerships in Implementation Research for Accelerated Results in Immunisation,” she underscored the importance of embedded implementation research in helping countries identify, test, and scale effective solutions in real-world contexts. “I was honoured to join key immunisation stakeholders and experts from across the continent in discussions that matter,” Dr. Ndwandwe shared. “These conversations highlighted the necessity for us to rethink how we collaborate, how we provide technical support to countries, and how we utilise evidence to accelerate the Immunisation Agenda 2030.” Dr. Ndwandwe also engaged in the Regional Immunisation Technical Advisory Group (RITAG) sessions, where global and regional experts reviewed progress, identified barriers, and made recommendations on various topics, including Mpox, RSV, polio transition, and African vaccine manufacturing. “We must transcend the view of research as something external to programmes. Instead, research should be integrated within the health system, co-created with country teams, and focused on the challenges decision-makers face on the ground,” she stated. A key focus was on Vaccine Implementation Science, which aimed at exploring how the immunisation ecosystem can better support countries through applied research. “These discussions reaffirmed the crucial role that implementation research plays in bridging the gap between evidence and impact,” Dr. Ndwandwe observed. Her participation underscores the SAMRC’s strategic commitment to implementation research, particularly through CSA, which advocates for generating and applying evidence to inform policy and practice. “At Cochrane South Africa, we are dedicated to ensuring that research is not only produced but also effectively used to strengthen immunisation delivery, improve coverage, and ultimately save lives,” she mentioned. As Africa moves toward achieving the targets of the Immunisation Agenda 2030, Dr. Ndwandwe’s message is clear: “To succeed, we must forge partnerships rooted in trust, align our efforts with national priorities, and ensure that implementation research becomes a routine aspect of how we approach immunisation.”  |
| CSA’s Ameer Hohlfeld participates in INGUIDE Training Programme |
|
Ameer Hohlfeld, Senior Scientist at the South African Medical Research Council’s (SAMRC) Cochrane South Africa (CSA) attended the International Guideline Training and Certification Programme (INGUIDE), which took place from the 21-26 June 2025 in Milan, Italy. INGUIDE is a flagship, ISO-certified training and certification program for guideline development, established by the Guidelines International Network (GIN), McMaster University, WHO, and implemented at Humanitas University. The course addresses global disparities in guideline quality, especially in low- and middle-income countries (LMICs), by training professionals to develop trustworthy, context-driven clinical and public health guidelines using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology and was attended by current and emerging guideline leaders, including five delegates from across Australia, Canada, Italy and Africa selected in partnership with WHO and sponsored by Humanitas University. The programe is aimed at those who would like to be formally recognised to form part of international guideline development groups. Learning outcomes included the Systematic use of research evidence in decision-making, best practices in guideline development (e.g., evidence synthesis, stakeholder engagement) and practical tools, including GRADE framework adaptation (“adolopment”) across diverse settings, with real-world insights gained from cross-country exchanges, highlighting both methodological rigor and contextual customisation. "Participating in INGUIDE at Humanitas was transformative—I gained not only in-depth methodological skills, but also a global network of proof-driven leaders committed to elevating guideline quality in diverse health systems", said Hohlfeld. With regards to future plans, he mentioned that he was looking forward to the continued rollout in the upcoming Course 3, and deepening competencies with a focus on building capacity in LMICs and expanding into related areas such as health technology assessment. “My ambition is to professionalise guideline methodology, enable mentorship, and secure funding for widespread and free certification”, concluded Hohlfeld.  |
| Cochrane South Africa presents Postgraduate Supervisors and Students Workshop in Evidence-Based Methods |
|
The Cochrane South Africa Training Working Group (TWG) serves to advance and encourage research excellence through building health research leadership and capacity to strengthen translation of research into public health impact., the TWG hosted a workshop focused on Evidence-Based Methods at Nelson Mandela University on the 26-27 March 2025. The workshop was facilitated by Prof Mark Engel and Mr Lorenzo Bennie. The programme was tailored to the needs of individual units or research groups, with this edition specifically for postgraduate supervisors and students, with emphasis on formulating an answerable question, searching for evidence using a targeted strategy, managing articles for data extraction, critical appraisal and synthesis of evidence using analysis software with the view of publication. The format of the workshop allowed interactive sessions; utilising examples brought forward by the participants’ research projects allowing for hands-on practical training with real-time problem solving and progress. Other noteworthy sessions included tools for assessing the risk of bias for various study designs, and a lecture on meta-analysis before culminating in finalizing a protocol and a write-up towards summarizing the evidence. The South African Cochrane Centre staff reminded the participants that ongoing mentoring following the initial sessions continues and remains available. Should you wish to receive invitations to attend these seminars or would like more information about these sessions please email cochranesa@mrc.ac.za. Learn more about Cochrane South Africa Learning & support initiatives | HERE
|
| CSA conducts Prevalence Estimates Review Workshop at 5th Cochrane Africa Indaba 2025 |
|
The 5th Cochrane Africa Indaba 2025 was hosted by Cochrane Kenya at the Argyle Grand Hotel in Nairobi, Kenya from 14-15 May 2025. The theme of this international evidence-based health care conference was "Advancing evidence synthesis for health decision-making in Africa: Promoting health equity and access", bringing together researchers, health professionals, policy-makers, national, regional and international stakeholders, evidence-based healthcare champions, Cochrane Africa collaborators, patient and community advocates, and students to discuss progress, opportunities, and the future of evidence synthesis in Africa. At the Indaba, Cochrane South Africa (CSA), hosted a Prevalence Estimates Review Workshop, which was facilitated by Prof Mark Engel (Unit Director) and Dr Kimona Rampersadh (Post-doctoral fellow). Prof Engel said “Prevalence studies employing a cross-sectional study design are useful for assessing disease burden or providing an understanding of the extent of a particular risk factor. Given the explosion in reporting of prevalence studies, rigorous syntheses of prevalence estimates have significant potential to assist stakeholders in resource allocation, personnel training, policy and decision making”. The learning objectives of the workshop included: An overview of prevalence reviews, framing a question, Critical appraisal tools, Analysis primer, using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) tool for prevalence studies together with Write up and interpretation sessions. “This workshop served to introduce participants to the distinctiveness of syntheses of estimates from prevalence studies and included a mixture of lectures and hands-on presentations”, added Prof Engel. Learn more about Cochrane South Africa | HERE |
| Cochrane South Africa facilitates EBHC Workshop at University of Venda |
|
The South African Cochrane recently conducted a two-day workshop on evidence-based healthcare (EBHC) research methods for staff and postgraduate students at the University of Venda. Hosted at the 2Ten Hotel in Limpopo from 17-19 March 2025, this hybrid workshop was facilitated by Prof Mark Engel, Ameer Hohlfeld and Lorenzo Bennie and was attended by post graduate students and staff members with the aim to provide participants with practical tools that can be incorporated into their research activities. The workshop covered a range of relevant topics such as refining your research questions, searching for relevant articles, critically appraising included articles and a meta-analysis primer. A key feature of this workshop was the interactive sessions where participants were encouraged to work on their research questions and to ultimately present their work for constructive feedback from peers, based on EBHC methods. Lorenzo Bennie said, “This workshop approach included many interactive sessions, where participants were given practical hands-on training, where they were able to work on their manuscripts. This deepened the participants' understanding of EBHC methods and contributed to a supportive learning environment.
|
| Cochrane SA runs research translation campaign to mark national Vaccination Week |
|
Marking national Vaccination Week from 24-30 April 2025, Cochrane South Africa ran a research translation campaign to highlight the findings from the systematic reviews they recently published on vaccination. Over the 3-day campaign, 7 national TV, radio and newspaper outlets were engaged with, including eNCA news, SA FM, Cape Talk, Power FM, Rise FM, Channel Africa, and The Citizen. In these engagements, scientists from Cochrane South Africa shed light on the complex social, political, and systemic reasons for vaccine hesitancy that were revealed by the reviews they led on views and practices around childhood and Human Papillomavirus (HPV) vaccination. They also highlighted the findings from their review on the effectiveness of mobile phone text message reminders to improve vaccine uptake and recall rates. They provided insights into how the public, health professionals, policymakers and others could use the findings from these different reviews to promote vaccination in ways that are more acceptable, sensitive and ultimately effective. According to Newsclip, the campaign generated R609 820 worth of positive publicity and reached about 2.3 million audiences.
|
| Dr Duduzile Nwandwe participates in the 12th EDCTP Forum |
|
Dr Duduzile Ndwandwe, a Specialist Scientist at Cochrane South Africa attended the 12th EDCTP Forum taking place from the 15–20 June 2025 in Kigali, Rwanda. The forum is a key platform for advancing Africa-led health research and innovation, bringing together a diverse set of stakeholders, including representatives from research institutions and universities, the larger scientific community, health care providers, governments, regional bodies, regulators, civil society, and public and private research and development partners. Dr Ndwandwe presented at the scientific symposium session “Improving Our Preparedness Capabilities for Global Response to Infectious Diseases”, with her talk entitled ‘Strengthening African Funder Coordination: The Establishment of the GloPID-R Africa Regional Hub,’ highlighting her teams work towards aligning funders to support regional outbreak response. She also participated on the Africa CDC panel on “How can Africa-led R&D transform health innovation on the continent?”, while also serving on the Meet-the-Expert speaker on clinical trial registration, drawing on the work done on the Pan African Clinical Trials Registry (PACTR) and Cochrane South Africa. Dr Ndwandwe said “It was gratifying to see PACTR mentioned in the opening plenaries as a testament to my team, who work tirelessly to keep the Registry going and relevant in the evolving research landscape. Strategic side meetings have been central to this Forum, strengthening ties with regional partners and advancing key initiatives under the GloPID-R Africa Hub and PACTR. These engagements continue to be crucial for establishing collaborative, African-led research ecosystems that foster transparency, preparedness, and lasting impact.” Learn more about Pan African Clinical Trials Registry | HERE
|




 Ms. Mathebula presented a poster titled: "Estimating the rate of vaccination and identifying associated factors influencing childhood vaccination uptake." This presentation marked the first sub-study of her PhD project, "Assessing routine childhood vaccination acceptance, hesitancy and refusal in Cape Town, Western Cape, South Africa." The research addresses key public health issues, directly aligning with the Congress's focus on "Social and Political Impacts of Vaccination and Vaccine Policy" and "Vaccine Hesitancy". Reflecting on the experience, Ms. Mathebula said that the inclusive nature of the congress proved invaluable for exploring the complexities of mixed methods design, as exemplified by the study she is currently conducting for her PhD. “The poster sessions, in particular, offered a significant opportunity to network, deliberate, and plan ways to advance science, encouraging insightful discussions on how to effectively combine quantitative and qualitative methods to address complex issues such as vaccine uptake and hesitancy”, added Ms. Mathebula.
Ms. Mathebula presented a poster titled: "Estimating the rate of vaccination and identifying associated factors influencing childhood vaccination uptake." This presentation marked the first sub-study of her PhD project, "Assessing routine childhood vaccination acceptance, hesitancy and refusal in Cape Town, Western Cape, South Africa." The research addresses key public health issues, directly aligning with the Congress's focus on "Social and Political Impacts of Vaccination and Vaccine Policy" and "Vaccine Hesitancy". Reflecting on the experience, Ms. Mathebula said that the inclusive nature of the congress proved invaluable for exploring the complexities of mixed methods design, as exemplified by the study she is currently conducting for her PhD. “The poster sessions, in particular, offered a significant opportunity to network, deliberate, and plan ways to advance science, encouraging insightful discussions on how to effectively combine quantitative and qualitative methods to address complex issues such as vaccine uptake and hesitancy”, added Ms. Mathebula.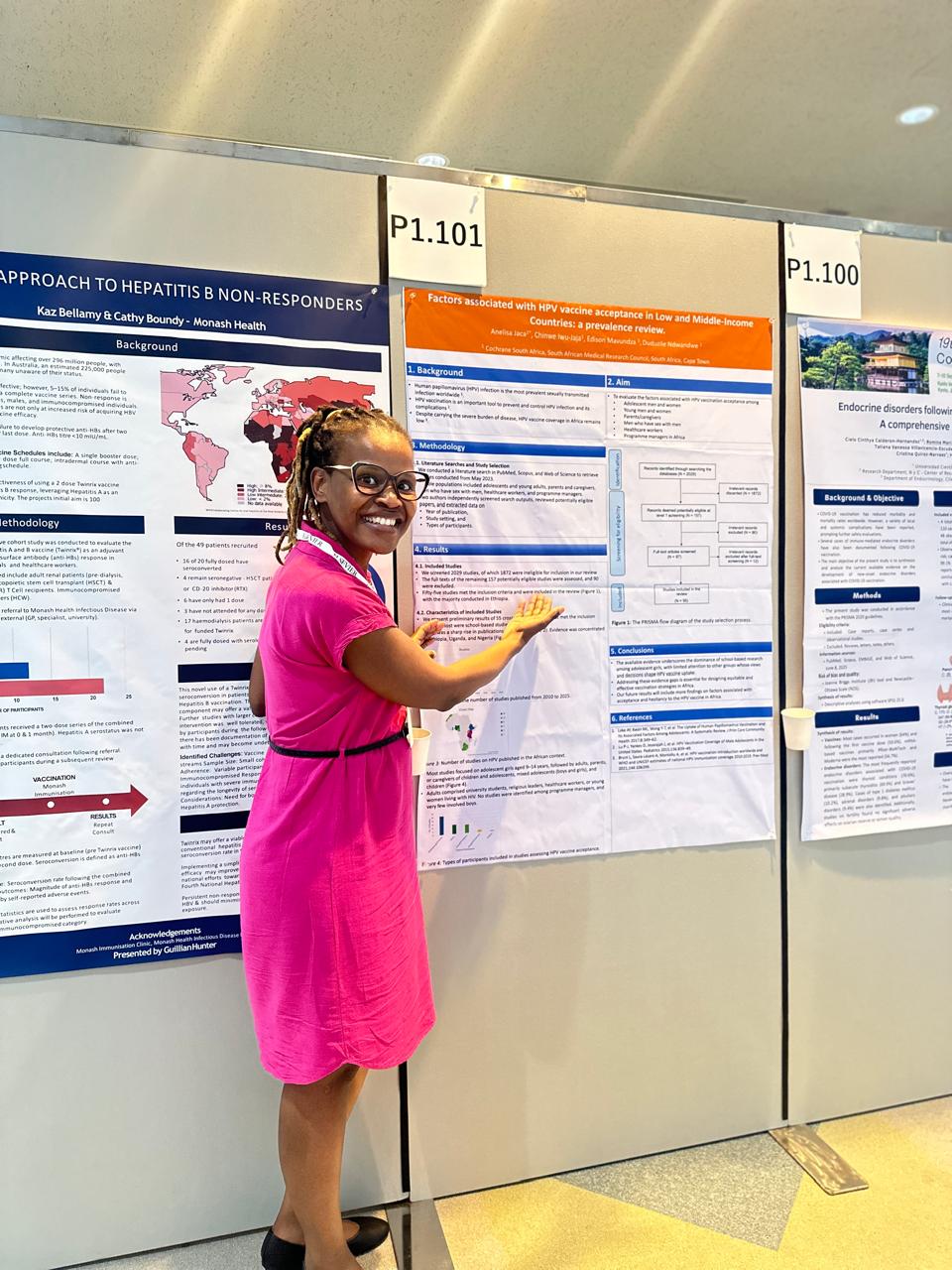 Dr. Anelisa Jaca presented a poster on the factors influencing HPV vaccine acceptance in low- and middle-income countries. Her review highlights important lessons for improving HPV vaccine acceptance in Africa. Current evidence is primarily based on school-based studies focused on adolescent girls, which leaves significant gaps in understanding the perspectives of other critical groups, including boys, men who have sex with men, parents and caregivers, healthcare workers, and program managers. These groups play crucial roles in influencing vaccination decisions, yet their views are underrepresented in the existing literature. Additionally, the concentration of studies in just a few countries, specifically Ethiopia, Uganda, and Nigeria, reveals a lack of geographic diversity, limiting the generalisability of findings across the continent.
Dr. Anelisa Jaca presented a poster on the factors influencing HPV vaccine acceptance in low- and middle-income countries. Her review highlights important lessons for improving HPV vaccine acceptance in Africa. Current evidence is primarily based on school-based studies focused on adolescent girls, which leaves significant gaps in understanding the perspectives of other critical groups, including boys, men who have sex with men, parents and caregivers, healthcare workers, and program managers. These groups play crucial roles in influencing vaccination decisions, yet their views are underrepresented in the existing literature. Additionally, the concentration of studies in just a few countries, specifically Ethiopia, Uganda, and Nigeria, reveals a lack of geographic diversity, limiting the generalisability of findings across the continent. The countdown is on for The South African Clinical Research Association (SACRA) Conference 2025, which will take place from October 1 to 2, 2025, at the CSIR Convention Centre in Pretoria. This year’s conference promises more space, extended networking, and unmatched opportunities for collaboration in the clinical research domain.
The countdown is on for The South African Clinical Research Association (SACRA) Conference 2025, which will take place from October 1 to 2, 2025, at the CSIR Convention Centre in Pretoria. This year’s conference promises more space, extended networking, and unmatched opportunities for collaboration in the clinical research domain.


